Details of the Drug
General Information of Drug (ID: DMHL7OB)
| Drug Name |
Uracil mustard
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Chlorethaminacil; Demethyldopan; Desmethyldopan; Nordopan; Uracillost; Uracilmostaza; Uramustin; Uramustina; Uramustine; Uramustinum; Aminouracil mustard; Uracil lost; Uracil lost [German]; Uracil mustard [USAN]; Uracil nitrogen mustard; ENT 50439; U 8344; CB-4835; SK-19849; U-8344; Uracil mustard (TN); Uracil mustard (USAN); Uramustina [INN-Spanish]; Uramustine (INN); Uramustinum [INN-Latin]; URACIL MUSTARD (500 MG) (FOR U.S. SALE ONLY); 2,6-Dihydroxy-5-bis(2-chloroethyl)aminopyrimidine; 2,6-Dihydroxy-5-bis[2-chloroethyl]aminopyrimidine; 5-(Bis(2-chlorethyl)amino)-2,4(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)-2,4(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)-2,4-(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)uracil; 5-(Di-(beta-chloroethyl)amino)uracil; 5-(Di-2-chloroethyl)aminouracil; 5-Aminouracil mustard; 5-N,N-Bis(2-chloroethyl)aminouracil; 5-[Bis(2-chlorethyl)amino]-2,4(1H,3H)pyrimidinedione; 5-[Bis(2-chloroethyl)amino]uracil; 5-[Di(2-chloroethyl)amino]uracil; 5-[Di(beta-chloroethyl)amino]uracil; 5-[Di(beta.-chloroethyl)amino]uracil; 5-[bis(2-chloroethyl)amino]-1H-pyrimidine-2,4-dione; 5-[bis(2-chloroethyl)amino]-2,4(1H,3H)-pyrimidinedione; 5-[bis(2-chloroethyl)amino]pyrimidine-2,4(1H,3H)-dione; 5-[bis(2-chloroethyl)amino]pyrimidine-2,4-diol
|
|||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||
| Structure |
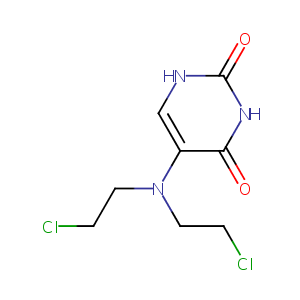 |
|||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 252.09 | ||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.5 | |||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Uracil mustard (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
